As published in Converting Quarterly in Quarter 1, 2022 by E.J. (Ted) Lightfoot, consultant, Ted Lightfoot LLC
Liquid coatings need to solidify before being wound into a roll. For ambient-temperature coatings, this requires drying, curing or a combination of the two. Here we discuss the basic configurations and the benefits and drawbacks of curing with and without drying.
Introduction and comparison of options
Drying is the removal of solvents (including water). Curing refers to chemical reactions that occur
within a coating – while it dries, after it dries or without drying. Drying, without any kind of curing, is the most common form of solidification. However, curing can produce thermoset materials and may greatly enhance the mechanical properties of the coating. Some ultraviolet (UV) and electron-beam (EB) coatings are solvent-free, which have environmental, safety and cost advantages. Examples
of product enhancements by curing are increased hardness, scratch-resistance and solvent-resistance.
The two most common types of curing reactions are crosslinking of polymers and free-radical polymerization. Well-known examples of cross-linking are two-part urethanes and epoxies; however, systems like aldehyde cross-linking of gelatin in photographic coatings illustrate the breadth of
chemistries (i.e., any reactive group) that can be used for curing.
Inside the dryer
Dryers used for simultaneous curing and drying are similar to dryers used for drying only. However, the configuration and temperature profile may be different. Aqueous drying profiles often start at high temperatures in the initial zones and bring the temperature down to avoid blisters. Most
organic solvent dryers start at low temperatures to avoid explosion hazards, then bring the temperature up to reduce the residual solvent level [1]. The rate of chemical reactions increases with concentration; thus, the rate of curing initially will increase as the coating dries out. As the coating
becomes dry, reactions become diffusion-limited and slow down near the end of drying. Thus, curing often is highest in the middle of the dryer and, given the usual Arrhenius behavior, it is not uncommon to see temperature profiles for curing that reach a maximum temperature nearer the middle of the dryer before tapering off to reduce residual stress in the coating.
Drying is a relatively slow process (residence times ranging from seconds to minutes). This contributes to the breadth of chemistries possible. But residence times for EB and UV curing often are a fraction of a second. Both UV and EB can be used to initiate polymerization reactions that with
polyfunctional monomers can create cross-linked network structures; however, EB also is known for its ability to cause chain scission and recombination that can lead to crosslinking in a wide range of polymers (without monomers present).
Polymerization process
Cross-linking can be done in a dryer, in an electron beam, under UV light or in a roll over a period of days to weeks. However, polymerization usually is confined to EB or UV curing. There are examples of pure thermally induced polymerization; however, from the perspective of the converting industry, these are exceptions and not the rule. For example, the Swedlow process can produce thermally
cured acrylic sheet and film ranging from a few centimeters down to roughly 150 microns in thickness [2]. UV-induced polymerization, on the other hand, is best suited for thin layers (like coatings).
UV curing requires at least one component of the mixture to have a high UV absorption (leading to free radicals that start the reaction). But high UV absorption means that in thick layers the reaction will initiate at the surface. This can be used to produce surface effects (e.g., crinkled surfaces),
but it limits the thickness for an effective bulk cure. Unlike UV light, electrons are not preferentially absorbed by an initiator; thus, EB has a much higher penetration depth than UV curing; it also does not require an initiator. Freedom from initiators frees the upstream portions of the process from having to control lighting to prevent premature curing. Also, by their nature, initiators tend to be unstable and can lead to storage issues (e.g., short shelf life).
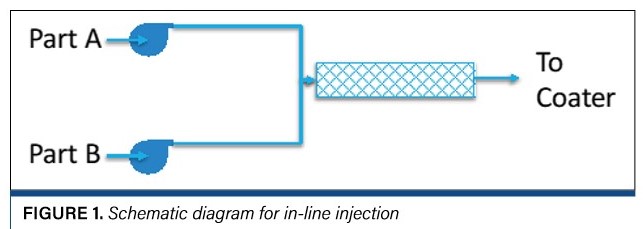
One way to minimize the sensitivity of reactive species – either in a dryer or a UV chamber – is to add photoinitiators or cross-linkers by in-line injection rather than in the mix area (see Figure 1). If the curing reaction occurs upstream of the coating head, the rheological properties [3] of the coating can change with time. In-line injection refers to pumping one part of a reactive system (usually an inert solution) to the coating head through an in-line (static) mixer where it is blended with the curing agent as the coating is delivered to the coating head. This allows much more reactive systems; however, it is useful only for pre-metered coating methods (slot-die, slide and curtain coating).
Electron-beam curing can be used for quite thick layers; however, the thicker the layer the more energetic the electrons need to be and the more expensive the curing system. Thus, most EB-curing systems for the converting industry are built for layers less than 50 microns thick, although systems for thicker layers are available.
UV-curing configurations
There are several configurations for UV curing. First, there are many kinds of UV light (energy) sources [4]. The wavelengths for these range from the deep UV (172 nm for excimer) to the visible violet light (405 nm for some LED curing) [5]. Second, oxygen inhibits UV curing; thus some UV-curing systems (particularly for thin layers) are nitrogen-purged [6]. Third, curing generally takes place in the absence of solvents or diluents.
For some systems (e.g., silicone release coatings), a very thin coating can be applied at 100% solids (e.g., by multiroll coating). But for many systems, the coating is made in a diluted form and dried prior to curing. Finally, curing can be quite exothermic. For high speed / short exposures, curing may be essentially adiabatic; however, for longer exposures, cooling rolls are required. The Tromsdoff – Norrish effect (runaway polymerization reactions that occur as the coating solidifies) provides additional incentive for cooling. Curing can lead to significant nonplanarities, including both thermally induced cockling and curl. Cockling generally is minimized by keeping the substrate held tightly against a cooling roller during curing (producing a machine-direction [MD] curl). While curl is not significant for thin, soft layers (e.g., silicone-release layers), it can be problematic for thicker layers. Minimizing curl requires minimizing the residual stresses in the coating.
Croll [7, 8] was the first to explain residual stress in coatings. As coatings dry or shrink (e.g., upon curing), the coating would like to shrink equally in all directions; however, because it is adhered to the substrate, it shrinks only in the direction perpendicular to the substrate. As long as the coating is liquid, no residual stresses are generated; however, once the coating solidifies, it takes stress to keep it stretched in the MD and transverse direction [TD].
Sources of residual stress
One source of residual stress is cooling of the cured solids. If the coating and substrate have different coefficients of thermal expansion, the product will act like a bimetallic thermostat (i.e., the curl will come and go and conceivably reverse as the product is heated and cooled). Thermal curl is best managed by keeping the coating as close to room temperature as possible during the curing reaction (i.e., use a chill roll). A second source of residual stress comes from drying, and this may be managed by adjusting the drying profile (often to push curing toward the end of the dryer). But a third source of residual stress comes from the curing reaction itself: Cured solids generally are denser than the uncured liquid. This source is in some ways unavoidable; however, it can be managed with formulation. The most successful strategy for managing residual stresses for curing was at the heart of the Swedlow process (the earliest roll-to-roll commercial curing process) shown in Figure 2 [9].
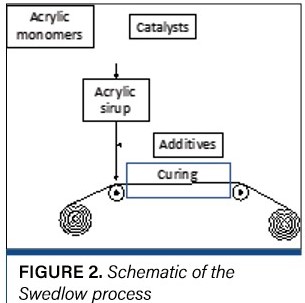
The key to the Swedlow process was to do as much reaction as possible prior to the coating to minimize both the heat of reaction and the volume change on curing. For acrylics, that means creating a “sirup” (with an “i” to distinguish it from liquids suitable for ingestion) of acrylic oligomers and monomers just before curing. However, the flowsheet of the Swedlow process shows the general approach taken by formulators of cured coatings ever since: The “sirup” can be made by dissolving premade polymers and oligomers that may be further polymerized or cross-linked in curing as well as other additives (including solid particles that will not shrink during the cure process) to minimize heating and density change on curing.
Summary
Drying is the most common way to create thermoplastic coatings, but curing is required to get thermoset materials. Curing in a dryer offers the broadest range of chemistries for cross-linking. UV curing is most widely used for either cross-linking or polymerization in thin layers and often is combined with drying. Electron-beam cross-linking and polymerizing usually eliminates the need for a dryer and has economic advantages. All three options have proven useful in practice, and formulators should be aware of all three as they develop new products.
Footnote and References
1. Concerns for “skinning,” coalescence, and optimization of product properties can also affect the profile.
2. L.E. Wolinski, “Film and Sheeting” in Encyclopedia of Polymer Science and Technology, New York, John Wiley and Sons, (1967)
3. E.J. Lightfoot, “Practical Rheology for the Web Coating Industry,” Converting Quarterly 2020 Q3, pp 53-58
4. Chris Davis, “The Emerging Trend of Hybrid Radiation Curing,” Converting Quarterly 2020 Q1, pp 82-87
5. Jennifer Heathcote, “The Path Forward in UV-LED Web Converting,” Converting Quarterly 2021 Q1, pp 76-82
6. Robert Rae, Ryan Turner, and Jennifer Heathcote, “Role of inert chambers and system maintenance in UV-curing of silicon-release coatings: Part 1,” Converting Quarterly 2021 Q3, pp 41-45.
7. Croll, S.G. (1979), The origin of residual internal stress in solvent-cast thermoplastic coatings. J. Appl. Polymer Sci., 23: pp 847-858. https://doi.org/10.1002/app.1979.070230319
8. Croll, S.G. (1979), “Residual stress in a solventless amine-cured epoxy coating,” J. Coatings Tech., Vol. 51, No. 659, pp 49-55
9. Ibid. 2.
E.J. (Ted) Lightfoot, principal consultant at Ted Lightfoot LLC, worked for DuPont for over 35 years in the photographic business, casting, coating and laminating fluoropolymer and optical films (as well as some emerging technologies that remain proprietary). His experience in R&D, plant support, as a Six Sigma Black Belt for Growth and in application development (helping customers develop processes and structured products) gives him a balanced perspective on what it takes to build a robust business. He also is a writer, speaker and instructor of short courses. Ted can be reached at 716-449-4455, email: TedLightfootLLC@gmail.com or www.TedLightfoot.com
Original article can be found online here.


